Advisory Committee for Therapeutics
The TREAT-NMD Advisory Committee for Therapeutics (TACT) is a unique multi-disciplinary group of international experts including: academic and industry drug development experts, representatives of patient foundations, and regulatory bodies. TACT takes on the challenge of evaluating the therapeutic potential of drugs across neuromuscular diseases including acquired and genetic motor neuron, neuromuscular junction, peripheral nerve, and muscle diseases. TACT aims to apply the learnings from previous experience to optimise drug design and development programmes by providing a comprehensive, confidential review containing constructive feedback and guidance.
TACT can address:
- Trial design.
- Drug formulation.
- Bioavailability and toxicology.
- Regulatory and marketing considerations.
- Recommendations including go-no-go milestones.
The TACT meets twice a year: once in the autumn and once in the spring. Applicants are asked to pay a contribution towards the meeting costs based on their stage of development and investment raised. Fees are scaled to reflect the level of support required to make TACT sustainable whilst factoring in the applicant’s financial capacity.
The TACT process is independent of any funding stream, but applicants often use its reports to support funding applications. A TACT review is NOT an endorsement by TACT or TREAT-NMD that a drug has any particular status in terms of regulatory or funding potential.
TACT news
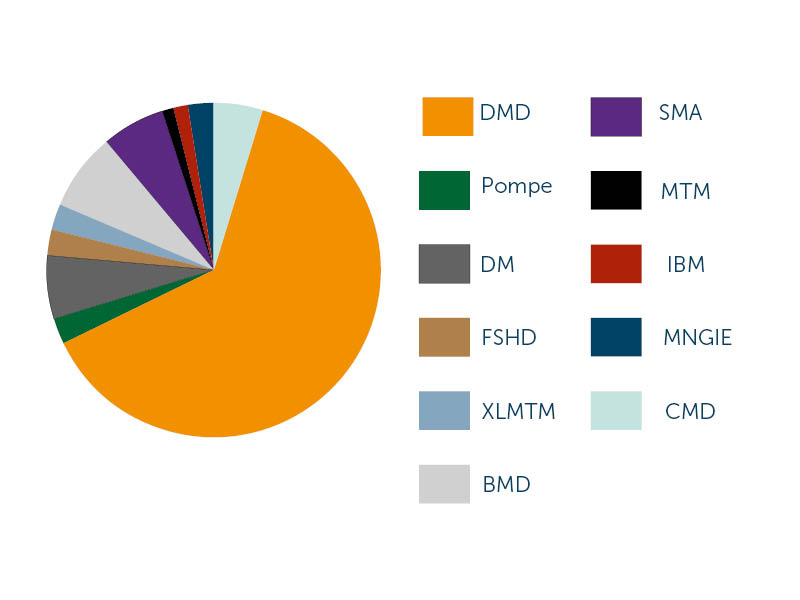
Past Applications by Disease Area
TACT publications
- Improving translatability of preclinical studies for NMD – click here
- TACT: an innovative de-risking model to foster orphan drug development – click here
- A decade of optimizing drug development – click here
Upcoming Advisory Committee Meetings

Summer 2025, Virtual Meeting
Full
July 29th 2025

Autumn 2025, Atlanta, USA
One Place Available
November 7th – 9th 2025

Spring 2026, European location TBC
Four Places Available
April 24th – 26th 2026

Ad Hoc Virtual Meetings
Please get in touch to find out if we can accommodate a virtual meeting: info@treat-nmd.com.
Virtual meetings are subject to approval and availability of the TACT core committee

